
The MCF has just installed a new inorganic mass spectrometry facility. The facility includes two new inductively couple plasma mass spectrometry (ICP-MS) systems: a Thermo iCAP RQ quadrupole ICP-MS for streamlined and high-throughput determinations of elemental concentrations and a Thermo Neoma multicollector ICP-MS with collision cell technology for the precise determinations of isotope ratios within a given sample. Each instrument can measure elemental variability in both dissolved aqueous samples as well as solids/minerals via laser ablation microsampling from a Teledyne Iridia laser ablation system.
Planned applications include:
(1) high-resolution measurements of Ca, Sr, Ba, Mg, and B elemental and isotopic variability in seawater and marine and terrestrial carbonates for paleoclimate reconstructions,
(2) (U-Th)/Pb dating and Hf isotope measurements to study the origin of critical mineral deposits, and
(3) the development of novel methods for increasing precision/accuracy and minimizing sample consumption during routine analyses of water quality and environmental contamination.The MCF welcomes users interested in these and other potential applications of this new facility to their scientific and engineering research to contact David Tavakoli (atavakoli6 @ gatech.edu)
To reserve the tool in SUMS, or to request training, please go here.
Coral Research
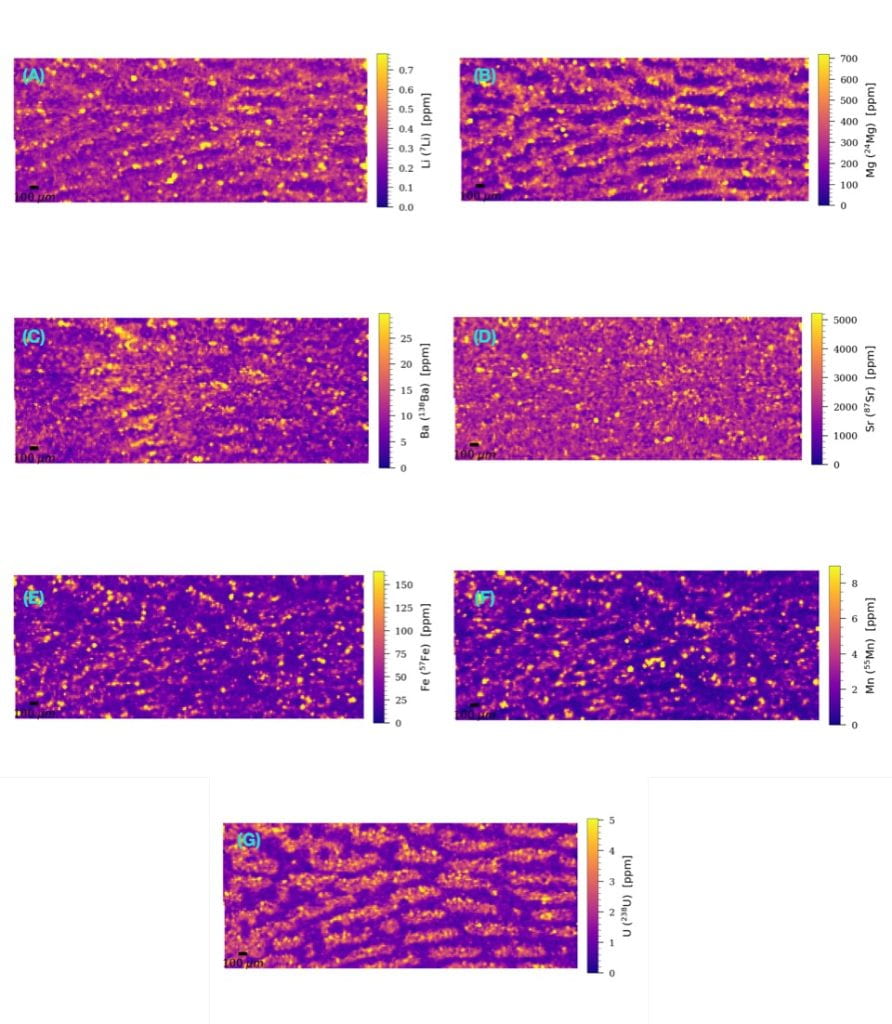
The new LA-ICP-MS facility in the GT Materials Characterization Facility is greatly assisting analyses of fine scale trace element incorporation into tropical coral skeletons. The Bolden group is ultimately interested in using data like this to better understand how these dynamic organisms respond to stressors – such as anthropogenic warming, ocean acidification, and others – in a rapidly changing world. Below, a skeletal sample of Siderastrea siderea (a.k.a Massive Starlet Coral) from Curaçao was collected and prepared for analysis. While a visual inspection of the sample produces limited information regarding the growth, morphology, and composition of the specimen, a laser-ablated raster of a small area of interest reveals incredibly interesting patterns in the distribution of trace elements throughout the calcium carbonate structure of the skeleton. “Under the surface” maps like these helps researchers think about (1) how biology and ambient seawater composition mediates the precipitation of coral skeletons on reefs, (2) where impurities in the skeletal structure tend to be located, and (3) how coral skeletons act as a time capsule capable of preserving signals of past environmental change.
Matrix Effects on Elements and Choice of Solvent
Most analysts prefer nitric acid (HNO3) matrices due to the solubility of the nitrates as well as its oxidizing ability and the relative freedom from chemical and spectral interferences as compared to acids containing Cl, S, F, or P. In addition, HNO3 is very popular in acid digestion sample preparations and is the current solvent mostly in use on the ICAP in the MCF.
The elements that are stable/soluble and commonly diluted in aqueous/HNO3 are shaded in red below:
- Os should never be mixed with HNO3 due to the formation of the very volatile OsO4.
- Cl is oxidized to molecular Cl2 which is volatile and adsorbs on plastic.
- Br and I are oxidized to molecular Br2 and I2 which adsorb onto plastic.
- Dilutions of Hg and Au in HNO3 below 100 ppm should be stored in borosilicate glass due to Hg+2 adsorption on plastic.
- Not soluble above concentrations of 1000 µg/mL.
- Trace levels of HCl or Cl- will form AgCl
 , which will photoreduce to Ag0.
, which will photoreduce to Ag0.
F denotes that the element can be diluted in HNO3 if complexed with F–.
Cl denotes that the element can be diluted in HNO3 if complexed with Cl-.
HF denotes that the element should have excess HF present when diluted with HNO3.
T denotes that the tartaric acid complex can be diluted in HNO3.
The use of hydrochloric acid (HCl) is the next most popular acid matrix. HCl is volatile and it is corrosive to the instrument and it’s electronics therefore, exposure should be kept to a minimum.
The elements that can be diluted in HCl are shaded in blue below: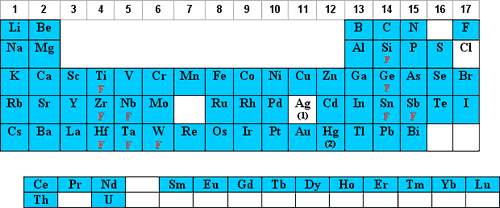
- Concentrated (35%) HCl will keep up to 100 µg/mL of Ag+ in solution as the Ag(Cl)X-(X-1) complex. For more dilute solutions, the HCl can be lowered such that 10% HCl will keep up to 10 µg/mL Ag in solution.
NOTE: The Ag(Cl)X-(X-1) complex is photosensitive and will reduce to Ag0 when exposed to light. HNO3 solutions of Ag+ are not photosensitive. - Parts-per-billion (ppb) dilutions of Hg+2 in HCl are more stable to adsorption on the container walls than are dilutions in HNO3.
F denotes that the element is more stable to hydrolysis if complexed with F–. In the case of Si and Ge the fluoride complex is generally considered a necessity.
Water at pH of 7
Dilutions in water at pH 7 are not as common for most elements but may be required to prevent chemical reactions of some of the compounds containing the element. Please note that solutions at pH 7 may support biological growth and therefore the long-term stability should be questioned.
Those elements that may have an advantage to being diluted in water at pH 7 are shaded in yellow below: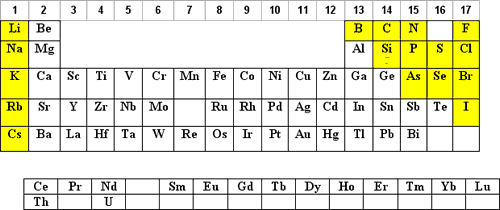
Hydrofluoric Acid Matrices
Hydrofluoric acid (HF) requires the use of HF-resistant introduction systems. These systems are more expensive than glass, have longer washout times, and give a larger measurement precision. However, there are times when the use of HF offers a major advantage over other reagents.
Those elements where an HF matrix may be optimal are shaded in green below: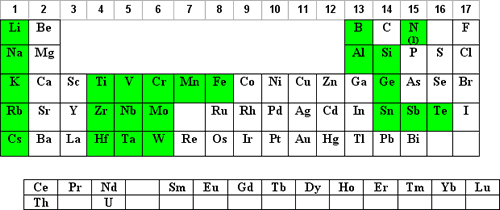
- HF is used for Si3N4 preparations and other nitrides.
Sulfuric Acid Matrices
Sulfuric acid (H2SO4) is commonly used in preparations and therefore added to standards in combination with other acids.
Elements that either benefit or comfortably tolerate the presence of H2SO4 are shaded in orange below:
- Dilutions of Hg and Au in H2SO4 below 100 ppm should be stored in borosilicate glass due to adsorption on plastic.
- Trace levels of HCl or Cl– will form AgCl
 , which will photoreduce to Ag0.
, which will photoreduce to Ag0.
F denotes that the element can be diluted in H2SO4 if complexed with F–.
Cl denotes that the element can be diluted in H2SO4 if complexed with Cl–.
HF denotes that the element should have excess HF present when diluted with H2SO4.
T denotes that the tartaric acid complex can be diluted in H2SO4.
Phosphoric Acid Matrices
Phosphoric acid (H3PO4) is not commonly used in preparations since it attacks glass, quartz, porcelain, and Pt containers at elevated temperatures (greater than 100 °C). However, the presence of 3PO4 will not adversely effect any of the elements at low µg/mL levels and below.
Documents
Standard Operating Procedure as of 12-05-2023
